Determination of Vitamin C Concentration by Titration Lab Report
Ensure there is a minimum of four samples to test in each group. An estimate of the dilution you will need to make in preparing samples d.

Experiment 5 Iodimetric Titration Of Vitamin C
1 mass of the sample used.

. The solution was made by adding 4 grams of vitamin C to 3 cups 750 mL of water. Enter your ctcLink ID and well send you a link to change your password. Add approximately 3 g KI and exactly 7500 mL of standard KIO 3 to each sample.
Experiment 4 Vitamin C Determination Page 3 2 3 4 323 ml at bottom of meniscus Reading the Buret You should take your readings at the bottom of the liquid meniscus and you should estimate to 001 ml as shown in the graphic to the right. L 0 gL The DV of vitamin C in the white cranberry juice sample is 100 then the amount of vitamin C per serving 0 L is. Vitamin C more properly called ascorbic acid is an essential antioxidant needed by the human body see additional notes.
X mg of vit. This method determines the vitamin C concentration in a solution by a redox titration with potassium iodate in the presence of potassium iodide. The aim of the experiment is to analyse the different concentrations of Vitamin C in fresh and commercial fruit juices.
Then report the average mg with error ie. The titration of Vitamin C with DCIP as your method of measurement. Demonstrate the technique for testing the samples.
The online lab procedure will give you some perspective. Ashenafi Asfaw Beyene Back Indirect Titration Vitamin C estimation by Back Titration Table1. Use the vitamin C solution for this experiment.
Determination of vitamin C in foods. Place 250 mL of the ascorbic acid solution which is 1 mgmL in a 125-mL flask. Part 1 Testing Vitamin C Solution 1.
We sometimes went 2 drops or 3 thinking it would reach the end point but sometimes we went over as evident by the color. To determine the quantity of Vitamin C ascorbic acid found in commercially available Vitamin C tablets by both acidbase and oxidationreduction titration methods. Determination of concentration of KMnO.
Assign each group to a set of equipment. Testing for Vitamin C- Part One Purpose. Lab s Conclusions Redox Titration Lab Google Sites.
This method determines the vitamin C concentration in a solution by a redox titration using iodine. C X 6 mg 0 g 0 g vit. 3 mL of Na 2 S 2 O 3.
Recall that the vitamin C is always colorless. Immediately titrate with thiosulfate until the solution has lost its initial reddish-brown color and has become pale yellow although it may be difficult to see these colors with other components of the tablets. A comparison of absolute accuracy between redox and acidbase titrations.
2 Our group sometimes placed the pipette on the sides of the graduated cylinder to drop DCPIP and it could have reacted with other chemicals. For the determination of Vitamin C in tablets. Calculate the mass of vitamin C per serving 250 mL of juice.
DETERMINATION OF VITAMIN C IN FRESH FRUITS AND VEGETABLES TABLE 1 Vitamin C content offresh fruits mg per 100g edible portion Dye-titration method 1 Microfluorometric method 2 Difference 2 - 1 Pear yellow Pyrus sinensis Apple red Pyrus mJ11us Grapes green Vitis vinifera Coconut young Cocosnucifera Applegreen Pyrus mJ11us. The amount of vitamin C in a sample will be determined by redox titration using the reaction shown in Scheme 1 between ascorbic acid and 2 6-dichloroindophenol DCIP. To test the level of vitamin C in a variety of fruit juices.
78 DCIP is used as the titrant because it should 1 only oxidize ascorbic acid and not other substances that might be present and 2 because it will act as a self-indicator in the titration. C then 0 L contains X mg of vit. Give each student a copy of Testing for vitamin C.
The method that will be used is a titration method where Vitamin C in fruit juices were titrated against aqueous sodium dichlorophenolindophenol with starch as an indicator. Check for overlap the same fruit or juice may be tested more than once by different groups giving multiple results for reliability. Ascorbic acid Vitamin C is a relatively cheap structurally simple water soluble organic acid--best known for being.
The standard deviation of Vitamin C in one tablet. At a minimum this plan must include. Vitamin C is one of the most important vitamins so reliable information about its content in foodstuffs is a concern to both consumers and quality control agencies.
As the addition of DCP proceeds the vitamin C in your sample becomes consumed. As the iodine is added during the titration the ascorbic acid is oxidised to dehydroascorbic acid while. The amount of volume in cm3 and concentration in M of KI KIO3 and H2SO4 which were used in the experiment of Vit C estimation.
Prepare a separate table. 78 DCIP is used as the titrant because it should 1 only oxidize ascorbic acid and. Calculate the mg of vitamin C in your 100 mL of juice sample as shown in the sample calculations below.
As long as some vitamin C remains in the sample the DCP will turn clear upon. 4 mol of Vitamin C in the sample of 5 mg of Vitamin C in the sample. Include in your report a discussion of the amount of vitamin C present in store-bought juice how it compares to the labels and if there is any change over time to the vitamin C content of the juice.
You may find reflections from the laboratory surroundings distracting as you try to take a reading. When iodate ions IO 3 are added to an acidic solution. Each juice is labeled to contain 130 of the daily requirement of Vitamin C per.
Calculate the number of juice servings that both a man and a woman would need to drink for their recommended daily allowance of vitamin C. 2 mL of KIO 3 used. Vitamin C more properly called ascorbic acid is an essential antioxidant needed by the human body see additional notes.
Testing the Standard Vitamin C Ascorbic Acid Solution 1. Determination of Ascorbic Acid in Vitamin Cpdf 9. This solution has more vitamin C in it than any of the fruit.
The DCP drops into a solution containing vitamin C the two chemicals react via a redox reaction and the indicator will go from red to clear. The amount of vitamin C in a sample will be determined by redox titration using the reaction shown in Scheme 1 between ascorbic acid and 2 6-dichloroindophenol DCIP. C 60 100 100 X 60 mg vitamin C per serving 0 L If 0 L contains 60 mg of vit.
Lab Report Oxidation and Reduction Experiment The. 6 mg of Vitamin C in the tablet. Redox Titration Of Vitamin C Lab Report Based On The Results Of Your Titrations Write The Balanced Half Reactions For The Reduction Of DCPIP By.
Vitamin c Estimation by backidiometric titration Mahindra UWC of India Chemistry lab report for back titration Name. Current state of method validation J Chromatogr A. A description how you will prepare your samples c.
However the heterogeneity of food matrixes and.
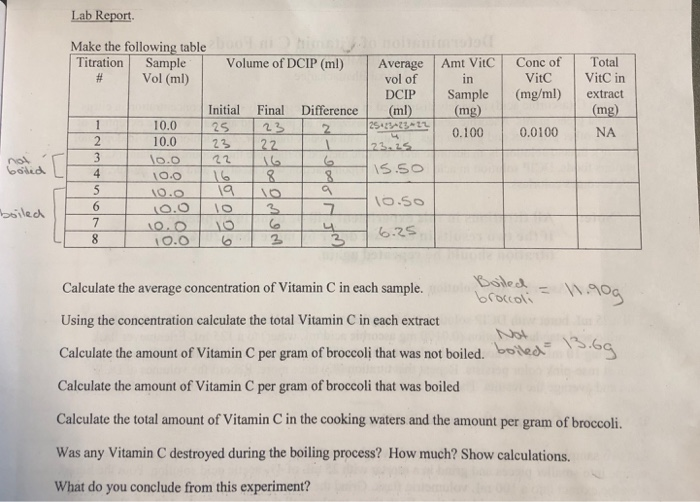
Solved Determination Of Vitamin C In Broccoli Lab Report Chegg Com

Dilmeet Sawhney Determination Of Vitamin C Concentration Lab Report Docx Determination Of Vitamin C Concentration By Redox Titration Lab Report Data Course Hero
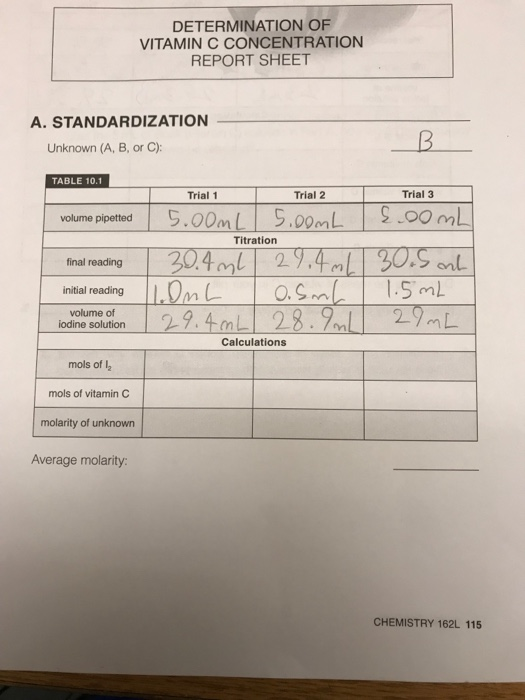
Determination Of Vitamin C Concentration Report Sheet Chegg Com
No comments for "Determination of Vitamin C Concentration by Titration Lab Report"
Post a Comment